| Gravimetric - determination of sulfate in fertiliser |
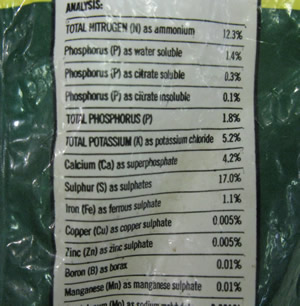 |
| Aim - To find the proportion of sulfate in a commercial fertiliser. |
|
Safety
- Safety goggles an lab coat
- Solutions of barium compounds are toxic
- Silver nitrate solutions stain skin and clothing
|
|
Materials
- Supply of fertiliser
- Solutions of 2 M HCl
-
Solution of 0.1 M AgNO3
- Solution of
0.5 M BaCl2
- Distilled water
- 2 X 100 mL beakers and one 600 mL beaker
- 10 mL and 20 mL measuring cylinders
- Filter paper and funnel
- Mortar and pestle.
- Electronic balance
- Bunsen burner, tripod, gauze mat and heat mat.
- Vacuum filtration unit.
|
|
Procedure
|
|
| Step 1 Using a mortar and pestle, grind to a fine powder a small sample of the fertiliser. Accurately weigh a 100mL beaker. Accurately weigh about 1.0 g of the fine powder and place it in a 100 mL beaker. Record the mass of the fertiliser and beaker. |
 |
| Step 2 Add about 50 mL of distilled water to dissolve all the sulfate in the fertiliser, then filter the mixture into a 600 mL beaker. Wash the residue thoroughly. |
 |
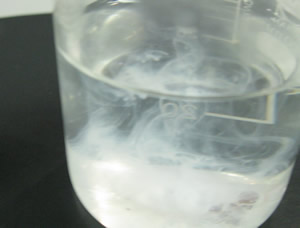
| Step 3 Add 3.00 mL of 2.0 M HCl to the filtrate and make it up to 200 mL with distilled water. Heat the solution over a Bunsen burner and bring it to boil. Remove the Bunsen burner and add 15.0 mL of 0.5 M BaCl2. A white precipitate of BaSO4 will form. |
 |
| Step 4 Return the beaker to the heat and continue to boil for another minute. Remove the beaker from the heat and allow to stand until the precipitate settles. Add a drop or two of 0.5 M BaCl2. If a precipitate forms add 3.0 mL of 0.5 M BaCl2 if no precipitate forms proceed to the next step. |
 |
| Step 5 Weigh and record the mass of a round filter paper. |
 |
| Step 6 Place the filter paper in the funnel of a vacuum filtration unit and wet with distilled water. |
 |
| Step 7 Filter the solution in the 600 mL beaker using the vacuum filtration unit making sure to collect al the precipitate in the filter paper. Wash the precipitate with distilled water. |
 |
| Step 8 After the final washing collect a sample of the filtrate and test it by adding one drop of 0.1 M AgNO3. If a white precipitate forms continue to wash the precipitate in the filter paper with distilled water. |
 |
| Step 9 Allow the filter paper to dry overnight and weigh on an electronic balance. |
 |
| Complete the table below |
|
| Mass of 100mL beaker |
|
| Mass of fertiliser and beaker |
|
| Mass of fertiliser |
|
| |
|
| Mass of filter paper |
|
| Mass of filter paper and barium sulfate |
|
| Mass of barium sulfate |
|
| |
|
| 1) Determine the percentage, by mass, of sulfate (SO4 2- ) in barium sulfate (BaSO4 ). Use percentage composition |
| 2) Using the percentage composition of sulfate in barium sulfate to determine the mass of sulfate in the precipitate. |
| 3) Find the percentage of sulfate in the fertiliser. |
| 4) Now determine the percentage, by mass, of sulfur in the fertiliser. |
5) How will the percentage of sulfur in the fertiliser that you calculated change:
-
if in step 2 you did not wash thoroughly the residue
- if in step 8 you did not test the filtrate with silver nitrate.
Offer an explanation to your answers above. |
6) Carbonates are found in the fertiliser and in the water when carbon dioxide dissolves.
a)
Why is HCl added at step 3) above?
b) Write a balanced equation for the reaction between H+ and CO32-
c) Why is the solution heated? d) What is the effect on the final percentage, calculated, of sulfur in the fertiliser if step 3) is not done? |
| 7) Why is it important for the precipitate to have a high molecular formula? |









